

Comprehensive, Customized Drug Product Development Services
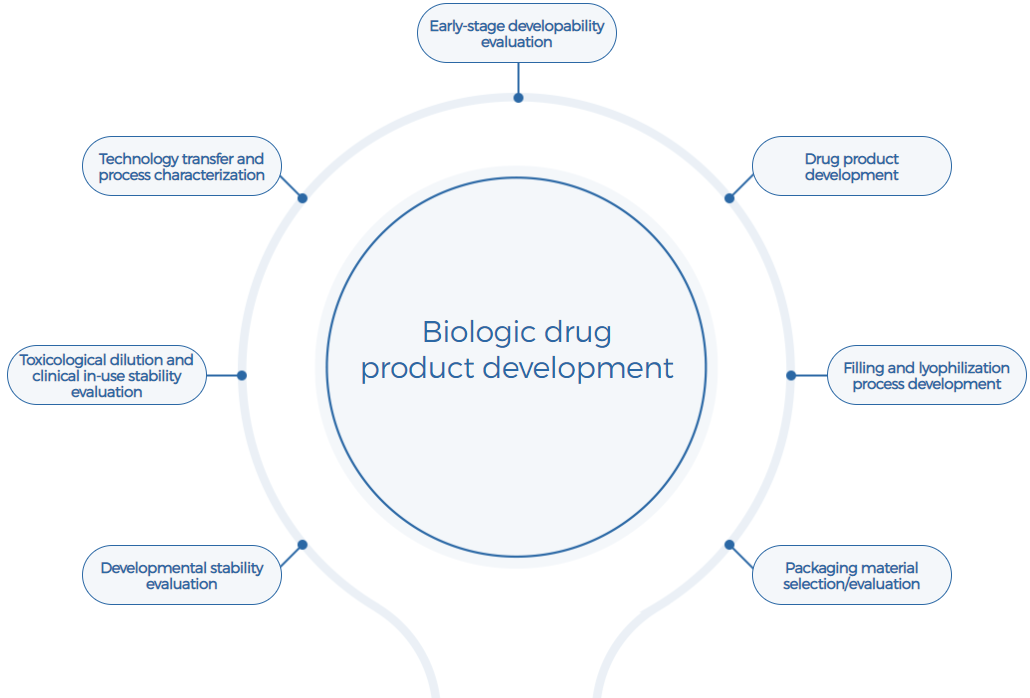
· Supports development of various conventional and challenging dosage forms, such as liquid and lyophilized products, multi-dose nasal spray, high-concentration liquid, etc.
· Covers various routes of administration, such as intravenous injection, subcutaneous/intramuscular injection, intranasal delivery, etc.
· Covers diverse molecular modalities, such as mAbs, bsAbs, fusion proteins, drug conjugates (ADCs, RDCs, AOCs, etc.), mRNA-LNPs, etc.
· Supports development at various stages, including pre-clinical phase, IND filing, clinical phases, and BLA filing
· Over 10 years of experience in biotherapeutics DP development