


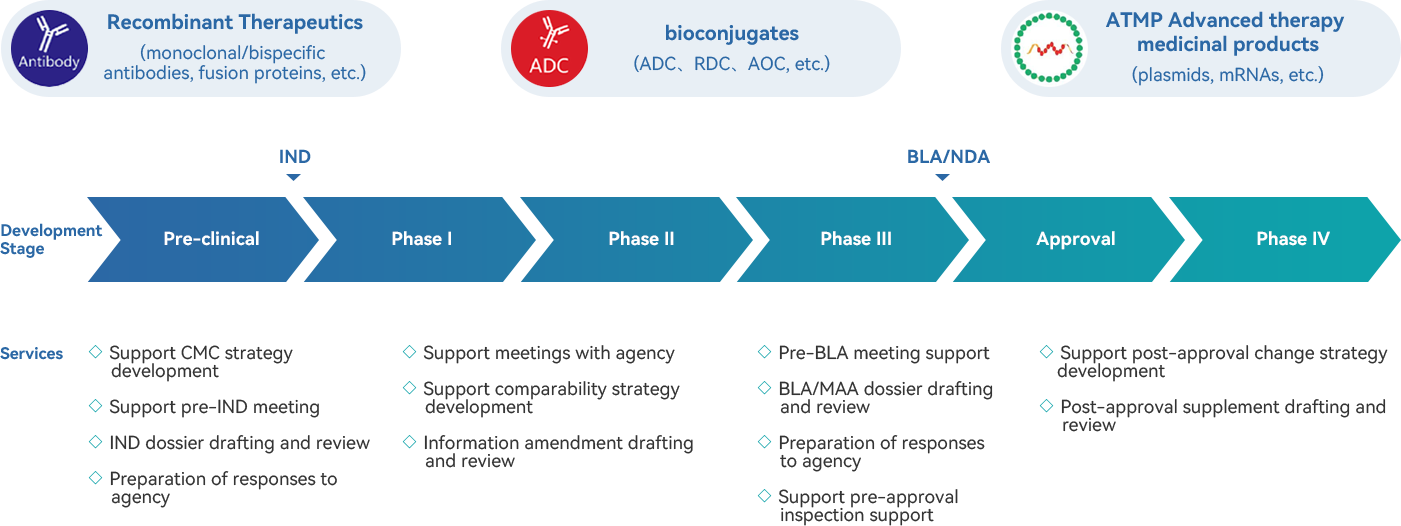
· Comprehensive CMC dossier drafting capacity, supporting the project progress of various modalities during different stages of the product lifecycle.
· Well-developed regulatory knowledge platform, regularly monitoring and understanding of regulation dynamics to ensure the continual compliance of projects.
· Abundant global resources, efficient communication channels with regulators worldwide, and close collaboration with industry associations have been established.
· Registration experience for multiple kinds of biologics, including eukaryotic and prokaryotic expression products, conjugation products, mRNA, etc.
· Extensive experience in dossier preparation for biologics IND, BLA, and PAC application